For the next 4 months, our team will be fully immersed in the Singapore market leveraging the 500 Startups’ network to multiply the number of strategic partnerships and accelerate international exposure of the company.
Healthcare research, being accelerated by the integration of technologies, faces a variety of challenges, including data privacy, process transparency, and ethical aspects. Seeing transparency as a cornerstone value in the future of life data exchange processes, in Q2 2019 Longenesis, in collaboration with Bitfury, has announced the launch of Dynamic Consent Management Toolkit (Longenesis Themis) solving the legal challenge of legitimate biomedical data utilization for clinical studies, allowing patients to be proactively involved in the clinical and research projects and providing transparency and auditability mechanisms, putting patients as a centric stakeholder of such process [1]. The tool is also tailored to comply with the Singapore Personal Data Protection Act and applicable to APAC region regulations.
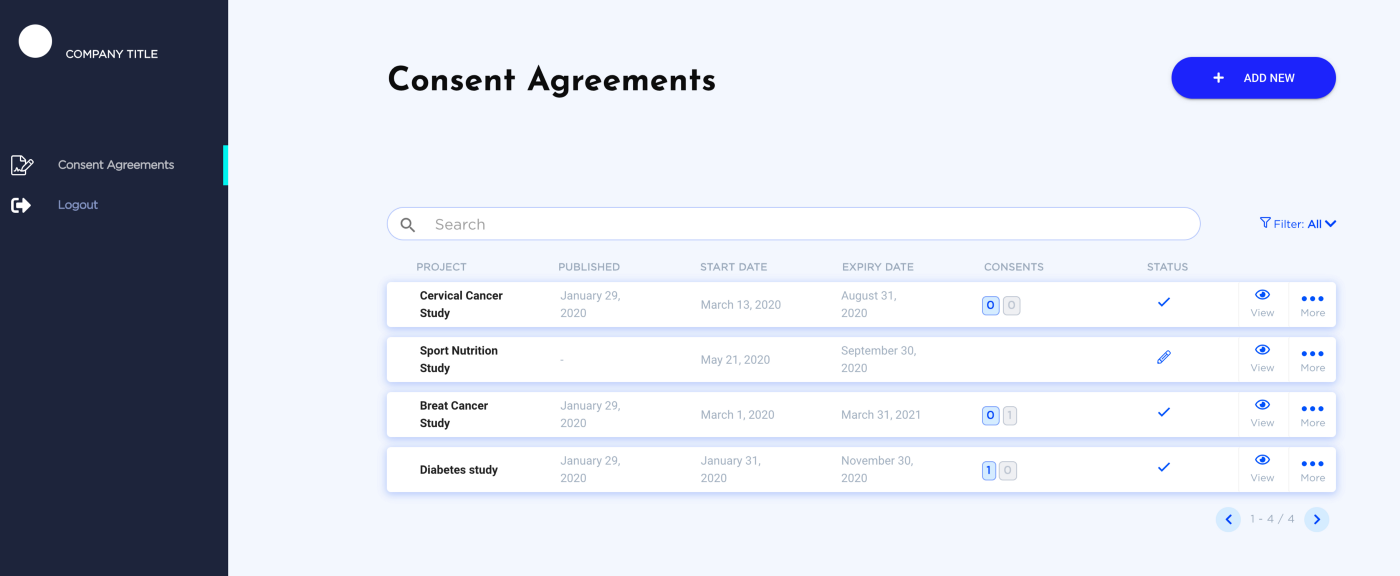
Inbound with the central product, Longenesis Proteus, company allows any clinical and/or research institution, patients association, biobank, and MedTech company to join the network and get connected with Clinical Research organizations and Pharma companies for proactive patient involvement.

Apart from its work connecting healthcare stakeholders and pharmaceutical industry, Longenesis has so far already conducted studies on Breast Cancer multi-modal risk calculation, Diabetes patients’ medical treatment monitoring and pharmacovigilance, being well-equipped to address patient needs in the APAC region [2].
References:
[1]: Bitfury and Longenesis Launch Medical Consent Solution, The Bitfury Group, April 4, 2019. Available: https://medium.com/meetbitfury/bitfury-and-longenesis-launch-medical-consent-solution-457bdf51fc9
[2]: Longenesis joins forces with Northern European oncologists, Labs of Latvia, January 22, 2020. Available: https://labsoflatvia.com/en/news/longenesis-joins-forces-with-northern-european-oncologists
